how to draw molecular orbital diagram of no
Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na. Molecular Orbital Diagram of NO.

Mathematics Origins Of Molecular Orbital Diagrams History Of Science And Mathematics Stack Exchange
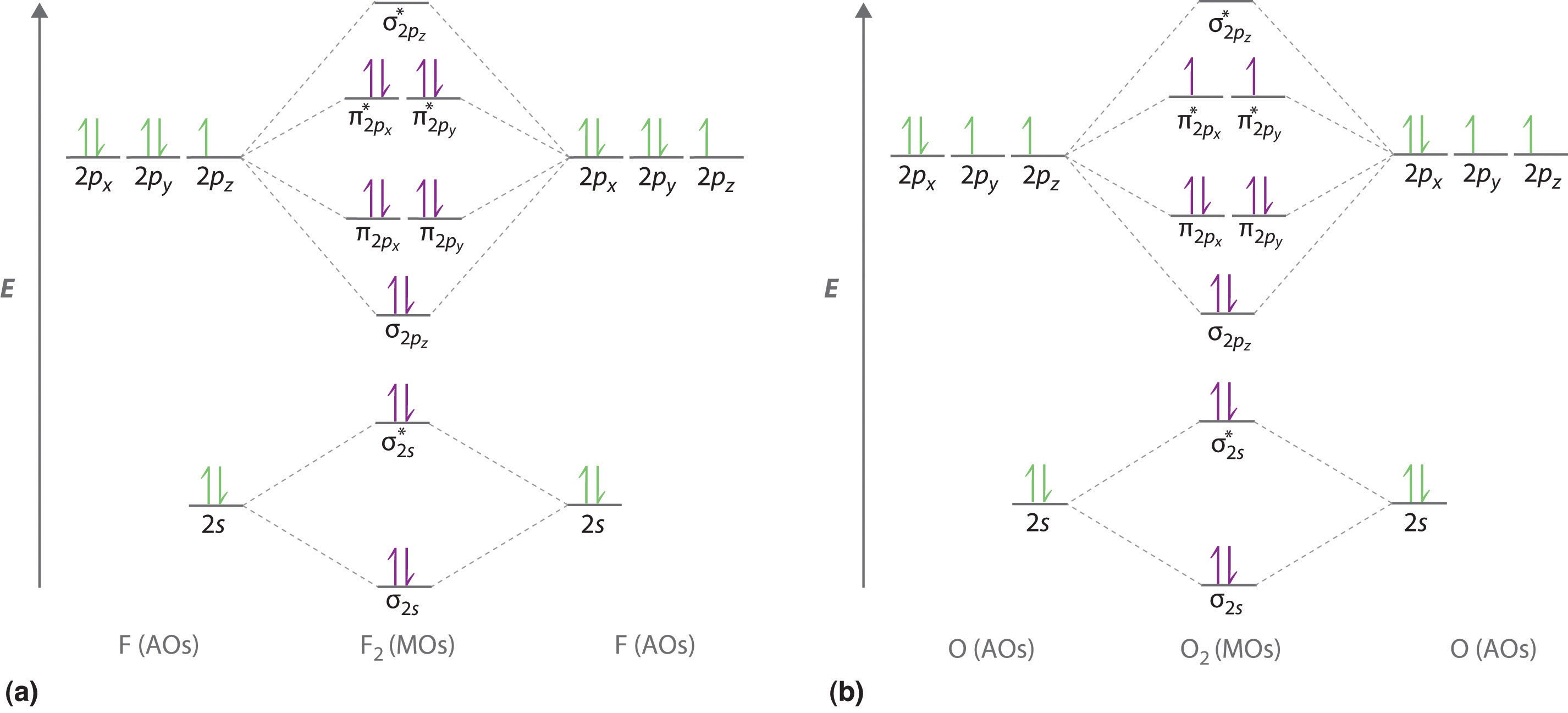
O2 kk σ2s 2 σ2s 2 π2p x 2 π2p y 2 π2p x 1 π2p y 1.

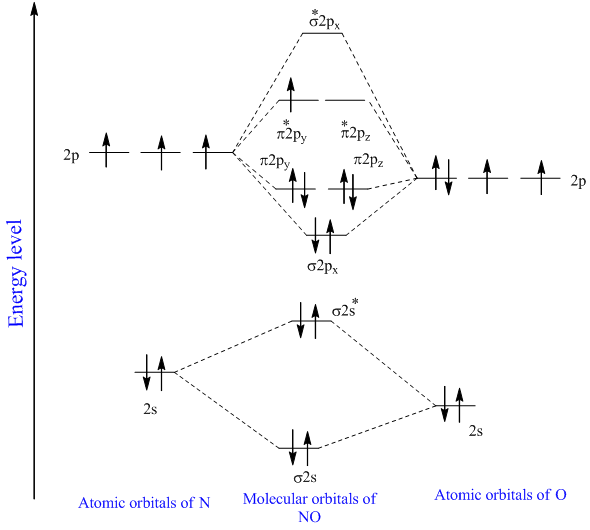
. The last electron is in fact in the 2b_1 antibonding MO so the bond order of NO has decreased by 12 relative to NO or CO. Assign x y z coordinates z axis is principal axis. Each horizontal line represents one orbital that can hold two electrons.
A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same. I was just wondering if the same applied for molecules with a. Draw the orbital diagram for the ion co2.
Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. 1 Stability of molecules in terms of bonding and antibonding electrons. If non-linear y axes of outer atoms point to central atom3.
N-O Hence the bond order of NO is either 25 or 35 depending on whether the last electron went into a bonding or antibonding MO. 2 So the formula to find bond order is Bond order dfrac12 Number of electrons in BMO Number of electrons in ABMO Bond order dfrac12 8 2 Bond order dfrac12 6 Bond order 3 - N_2 molecules are diamagnetic with no unpaired electrons. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram.
This image shows the molecular orbitals of nitric oxide and the types of bonds present. BO 12 bonding e- - antibonding e- 122222 - 21 colorblue25 And this should make sense because NO is isoelectronic with CO which has a bond order of 3. For a diatomic molecule the atomic orbitals of one atom are shown on the left and those of the other atom are shown on the right.
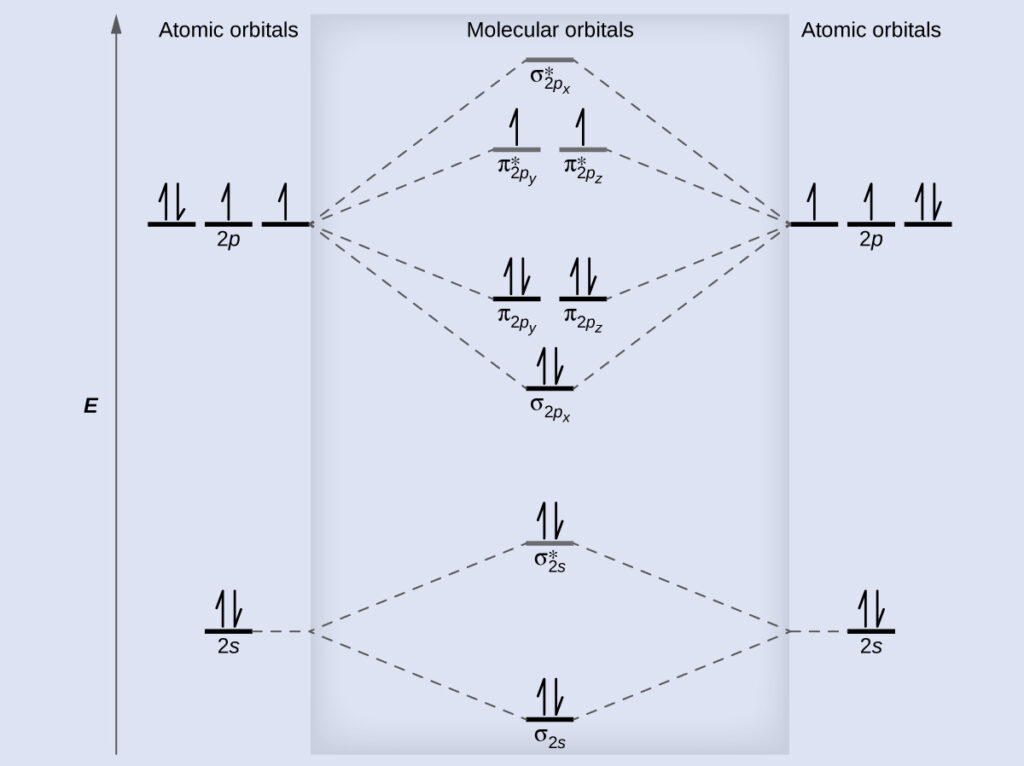
σ 1 s 2 σ 1 s 2 σ 2 s 2 σ 2 s 2 Π 2 p x 2 Π 2 p y 2 σ 2 p z 2. Previous article Molecular Orbital Diagram of CO. It is analogous to the atomic orbital energy diagram which goes 1s 2s 2p 3s.
Answer 1 of 2. Molecular orbital diagram of no. Number of electrons in antibonding orbitals.
Relationship between electronic configuration and Molecular behaviour. Draw the MO for O 2. For more informative Chemistry Lessons Subscribe DIGITAL KEMISTRY.
What will be the molecular orbital diagram for nitrite ion. Depending on if it is a homonuclear case where the bonding atoms are the same or a. 8 - Drawing Molecular Orbital Diagrams Flux Science.
Hence the electronic configuration of N 2 ion will be. Determine point group of molecule if linear use D2h and C2v instead of Dh or Cv 2. Click bellow CHANNEL LINK to subscribehttps.
Next article Qualitative and Quantitative Analysis Organic Chemistry. The MO diagram of NO is. 1 If N b Nathe molecule is stable because greater number of.
In contrast to VSEPR and valence bond theory which describe bonding in terms of atomic orbitals molecular orbital theory visualizes bonding in relation to molecular orbitals which are orbitals that surround the entire molecule. A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals method in particular. The molecular orbital diagram for Nitrogen dioxide NO₂ should loo.
In the link above chem_mod said it was best to account for the negative charge of CN- by placing an extra electron on the nitrogen since it is more electronegative. Molecular Orbital MO Theory is the final theory pertaining to the bonding between molecules. Molecular orbital diagram of N 2 BO Nb-Na 10-4 3 Since all the electrons in nitrogen are paired it is diamagnetic molecule.
So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0. MAKE SURE TO SUBSCRIBEThis video puts emphasis on molecular orbital diagrams a fundamental way of understanding why Diels-Alder chemistry works. The purpose of MO theory is to fill in the gap for some.
I think you can safely assume to start off with the molecular orbital diagram of the Nitrite anion NO₂ and then remove an electron from it. Molecular Orbital Diagram of NO. So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each.
Molecular Orbitals for Larger Molecules 1. 8 - Drawing Molecular Orbital Diagrams. The bond order is Figure The molecular orbital energy-level diagram for both the NO and CN-ions.
Find the characters of the reducible representationfor the combination of. Molecular Orbital Diagram For No Download Scientific Diagram Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. Draw the mo diagram for n2.
5 4 1 Molecular Orbital Theory Chemistry Libretexts

Delocalized Bonding And Molecular Orbitals
Explain The Mo Diagram For No Molecule Sarthaks Econnect Largest Online Education Community

8 4 Molecular Orbital Theory Chemistry
What Is The Molecular Orbital Diagram For No Quora
What Is The Molecular Orbital Diagram For No Quora

Tikz Pgf Molecular Orbital Diagrams In Latex Tex Latex Stack Exchange
What Is The Molecular Orbital Diagram For No Quora
Chem 2303 Supplementary Problems

Molecular Orbital Diagram For No Download Scientific Diagram
Molecular Orbital Diagrams Bond Order And Number Of Unpaired Electrons Chem Textbook
What Is The Molecular Orbital Diagram For No Quora
Solved Chapter 5 Problem 7p Solution Inorganic Chemistry 5th Edition Chegg Com

Mo Diagram Overview How To Draw Mo Diagram And Solved Example Along With Faqs

File Nitric Oxide Mo Diagram Svg Wikimedia Commons

Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com

Construct Molecular Orbital Diagram And Determine Unpaired Electrons In O2 O2 Bn No Study Com
